The measurement of cell characteristics in a population is crucial for many aspects of medical research. One of the main techniques that are utilized by scientists to perform this task is flow cytometry. This article will discuss the technique of hydrodynamic focusing, which is part of the field of flow cytometry.
Cells imaged in flow cytometer. Image Credit: Kateryna Kon / Shutterstock.com
What is flow cytometry?
Flow cytometry is a technique that is used to detect and measure the chemical and physical characteristics of a cell population. This technique, which was first used in the 1950s, can also be used to measure a population of particles.
A flow cytometer works by passing a population of cells through the equipment in a flowing fluid screen. The system then views, counts, and analyzes the cells present in the sample and defines their characteristics. Modern flow cytometers can count up to 10,000 cells per minute as they pass by the instrument’s laser.
Because of the speed and ability of flow cytometers to analyze individual cells in a sample, cell biologists are granted the statistical power to rapidly count and analyze millions of cells. Notably, flow cytometers are limited in their ability to detect morphological differences and subcellular localization; therefore, microscopy is better suited for those applications.
Flow cytometers have several applications, which include:
- Detection and measurement of protein expression throughout the entire cell.
- Detection and measurement of genetic material including ribonucleic acid (RNA), microRNA, messenger RNA (mRNA) transcripts, and long-coding RNA (lncRNA).
- Detection and measurement of cell health status.
- Detection and measurement of cell cycle status.
- Identification and characterization of distinct subsets of cells in a heterogeneous sample.
An overview of the flow cytometer system
The three main components of a flow cytometer include a fluidic system, optical system, and various electronics. The fluidic system is where the sample is transported from the sample tube to the flow cell.
The optical system of a flow cytometer consists of lenses, filters, and excitation light sources, such as lasers. The detection system that generates the photocurrent is another part of this component. Thirdly, the electronics of the flow cytometer digitize the photocurrent and save it for subsequent analysis.
Flow cytometers are different from cell sorters, which can sort cells and recover each subset for further use post-experiment. Flow cytometers do not have cell sorting capabilities.
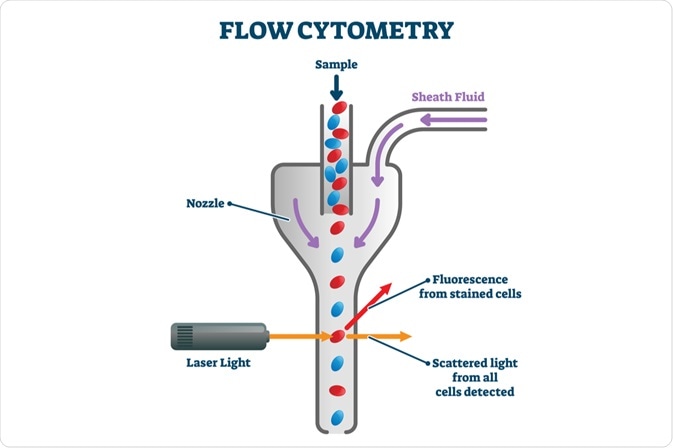
Image Credit: VectorMine / Shutterstock.com
Hydrodynamic focusing
Despite the utility of flow cytometry, these systems are limited in their ability to produce highly accurate results. However, several techniques can be used to solve this problem, which includes hydrodynamic focusing.
One reason that the data obtained in a flow cytometer may be inaccurate is that it is difficult to create narrow enough tunnels to pass the cell or particle populations through. For the best results, tubes must have a diameter of the magnitude of micrometers, with a length of several millimeters.
Hydrodynamic focusing solves this issue by using fluid dynamics. In this method, the walls of the sample tube are built up from liquid. The tube itself is wide, with a diameter of several hundred microns, and is made of glass or plastic.
A “sheath flow” of fluid is then pumped into a capillary and through the tube. By injecting the sample into the middle of the sheath flow, differences in density or velocity of the two liquids allow the cells to flow through the tube separately.
By using this fluid sheath, cells or particles can be confined and individually analyzed. The rate of the central fluid changes as the sheath fluid moves around the flow cell. A parabolic flow profile is achieved, with rates higher at the center and zero at the chamber walls.
Under laminar flow, which is the optimal condition for hydrodynamic focusing, the cell suspension does not mix with the sheath fluid due to differences in pressure. Cells are forced to pass in a single file through the optical laser beam.
With hydrodynamic focusing, rapid mixing at low pressures can be achieved with minimal sample consumption. Sheath pressure is constant, whereas the sample pressure can be adjusted. Without the hydrodynamic processing step, it is difficult to achieve accurate single-cell resolution.
3D hydrodynamic focusing
One problem inherent in 2-dimensional (2D) hydrodynamic flow chambers is the tendency for cells to adhere to the surface of the channel. This can lead to blockages of the sampling channel by these adherent cells.
Three-dimensional (3D) hydrodynamic focusing techniques circumvent this problem. A 2011 paper proposed a new 3D technique that utilizes a simple and integrated hydrodynamic focusing microfluidic device. This device consists of three sheath-flow channels that are located on either side and below the sampling channel. This forms a soft fluid wall that can prevent cell adhesion to the chamber surface,
When this system was used, labeled cells were hydrodynamically focused three-dimensionally and smoothly and individually introduced into the cross-section. The rate of introduction into the separation channel was a maximum of 151 cells min-1. By applying an electric field to the separation channel, the cells were introduced and rapidly lysed within 400 milliseconds (ms). Sodium dodecylsulfate was added to the sheath-flow solution.
To evaluate the system, reduced glutathione (GSH) and reductive oxygen species (ROS) in single HepG2 cells were analyzed. The average throughput rate for analysis in single cells was 16-18 cells min-1.
In addition to the system discussed here, several other 3D techniques have been proposed over the past decade to solve the problems with 2D hydrodynamic focusing.
Conclusion
The principles of hydrodynamic focusing are fundamental to flow cytometry. Without this tool, acquiring accurate data on single cells in a population is extremely difficult.
Hydrodynamic focusing is well-established in the field of flow cytometry. There are some newer techniques that have also been developed to enhance the sensitivity of flow cytometers, such as acoustic-assist focusing, which may start to replace hydrodynamic focusing as a future standard in flow cytometry.
References
Further Reading