Calreticulin is an essential protein present in the endoplasmic reticulum of cells, which is capable of binding to calcium and has some vital nuclear functions as well as functions that are wholly independent of the endoplasmic reticulum.
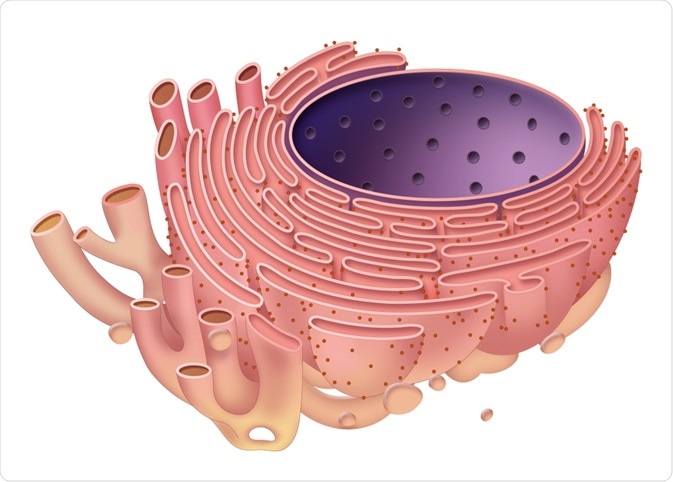 Image Credits: Aldona Griskeviciene / Shutterstock.com
Image Credits: Aldona Griskeviciene / Shutterstock.com
It is used by the body to regulate the transcription of genes via this protein’s ability to attach to protein motifs inside nuclear hormone receptors of sterol hormones, within the DNA-binding domain.
Calreticulin has previously been shown to prevent the binding of VDR, as well as displaying actions that regulate levels of osteocalcin expression in an in vitro setting. For a long time, the functions of calreticulin were studied by a comparatively small group of researchers that were interested in defining the role of calcium ion signalling in the cellular response to significant stressors.
These studies quickly came to identify that in the cellular endoplasmic reticulum, calreticulin binds not only to calcium ions, but was also found to interact with several ER protein. In recent studies, calreticulin levels have been associated with cancer development (tumorigenesis), adaptation and differentiation of cancer cells, and cancer metastasis.
Why is calreticulin important?
Calreticulin was initially identified as the Ca2+ binding protein in 1974, with later evidence indicating that this protein has a significant impact on the development of different cancers. More in-depth research on the effect of calreticulin on the formation of tumors, as well as tumor progression, shows that these effects could well depend on cell types and clinical stages of cancers.
Cell surface calreticulin has been shown to provide a signal to promote the phagocytic uptake of harmful cancer cells via the immune system, and several reports have presented that the manipulation of the levels of calreticulin within the body can profoundly affect the proliferation of cancer cells and angiogenesis, as well as cancer cell differentiation.
In addition to this, interactions between integrins and calreticulin have been previously described during the process of cell adhesion, which is a vital process for the metastasis of cancers.
Can calreticulin cause disease?
The intrinsic disorder predisposition of calreticulin mutation disorders (CALR) was observed in two independent studies that utilized multiparametric computational analyses: RONN and PONDR-FIT disorder predictors, or a selection of four different algorithms from the IUPred web server and the PONDR family. These different analyses later revealed that the N-domain is now expected to be mostly ordered, whereas the C-terminal tail and P-domain of the C-domain can be highly disordered.
These computer-based tools were selected for these analyses because of a variety of particular features, such as specificity and higher accuracy. The consensus disorder pre-disposition of human CALR diseases were also evaluated in these studies by analyzing disorder profiles of this individual predictor software.
The utilization of consensus for the evaluation of this intrinsic disorder was driven by previously ascertained empirical observations that the performance of these predictive software types can be greatly increased via this approach, compared to using a single predictor alone.
Furthermore, the observation of mutations within exon 9 of the CALR gene in the majority of cases of primary myelofibrosis and JAK2 wild-type essential thrombocythemia was a very sudden discovery, providing precious insight into the pathogenicity of these dangerous and rare diseases.
This surprising breakthrough in genetic research dominated the research of myeloproliferative neoplasms in the mid 2010’s and has since provided investigators with a new perspective on genetic diseases and the effects that these genes have upon them.
All of these studies have therefore concluded that abnormal calreticulin levels are definitively related to harmful outcomes in different cancer types. Furthermore, extensive evidence has proven that calreticulin participates in many different cellular functions both outside and inside the lumen of the endoplasmic reticulum.
It has since been observed that the two most vital functions of calreticulin are the chaperoning of proteins and maintaining calcium ion homeostasis. Additionally, further studies being conducted in more recent years are steadily beginning to indicate that non-endoplasmic calreticulin can also play essential roles during cancerous tumourigenesis.
The most important calreticulin-mediated mechanisms have been found to regulate cancer cell adhesion via interaction with cellular integrins. All of this since discovered evidence, combined with connections to extracellular matrices, show that the activation of integrins has been found to significantly impact the cytoskeletal dynamics within cells via several cytoplasmic-binding proteins.
Sources
Varricchio L. et al. (2017). Calreticulin: Challenges Posed by the Intrinsically Disordered Nature of Calreticulin to the Study of Its Function. https://doi.org/10.3389/fcell.2017.00096
Lu Y. et al. (2015). Functional Roles of Calreticulin in Cancer Biology. http://dx.doi.org/10.1155/2015/526524
Silver J. et al. (2018). Vitamin D and the Parathyroids. https://www.sciencedirect.com/book/9780128099650/vitamin-d
Further Reading