Stromal cells – also known as mesenchymal stem cells (MSCs) – are non-hematopoietic, multipotent, self-renewable cells that are capable of trilineage differentiation (mesoderm, ectoderm, and endoderm). The pluripotency and immunomodulatory features of MSCs mean that they are an effective tool in cell therapy and tissue repair.
Skip to:
- What defines a stromal cell?
- Sources of MSCs
- Isolation and culture of MSCs
- Expression of cell surface markers
- Capacity for long-term in vitro culturing of MSCs
- Immunomodulatory effects of MSCs
 Vshivkova | Shutterstock
Vshivkova | Shutterstock
Mesenchymal stem cells are easy to isolate and culturally expandable in vitro for long periods of time without losing their characteristics. They are able to trans-differentiate into ectodermal cells and endodermal cells. Moreover, due to their abundance in the adult body, research on these cells does not require ethical approval. MSCs are also safer than iPSCs, with no risk of teratoma formation. This makes them ideal candidates for cell therapy.
What defines a stromal cell?
The International Society for Cellular Therapy provides the following guidelines on mesenchymal stem cells:
- The cells should demonstrate plastic adherence.
- They should express specific cell surface markers, such as cluster of differentiation (CD) 73, D90, CD105, and lack the expression of CD14, CD34, CD45 and human leukocyte antigen-DR (HLA-DR).
- They should be able to differentiate in vitro into adipocytes, chondrocytes, and osteoblasts.
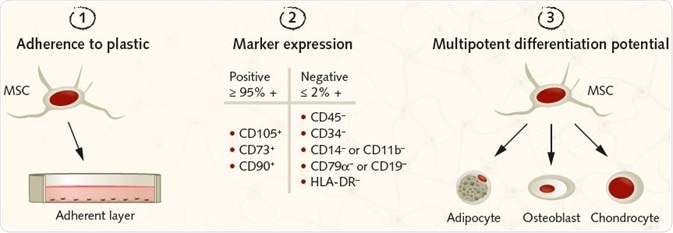 Figure 2. Summary of the ISCT criteria for identifying MSCs for research purposes. Image Credit: PromoCell GmbH. (1) MSCs must be plastic-adherent under standard culture conditions. (2) MSCs must express the surface antigens CD105, CD73, and CD90. A lack of expression of hematopoietic antigens (CD45, CD34, CD14/CD11b, CD79a/CD19, HLA-DR) is recommended, along with a minimum purity of ≥95% for CD105, CD73, and CD90 positive cells and ≤2% expression of hematopoietic antigens. (3) MSCs must be shown to be multipotent and be able to give rise to adipocytes, osteoblasts, and chondrocytes under the standard in vitro tissue culture-differentiating conditions.
Figure 2. Summary of the ISCT criteria for identifying MSCs for research purposes. Image Credit: PromoCell GmbH. (1) MSCs must be plastic-adherent under standard culture conditions. (2) MSCs must express the surface antigens CD105, CD73, and CD90. A lack of expression of hematopoietic antigens (CD45, CD34, CD14/CD11b, CD79a/CD19, HLA-DR) is recommended, along with a minimum purity of ≥95% for CD105, CD73, and CD90 positive cells and ≤2% expression of hematopoietic antigens. (3) MSCs must be shown to be multipotent and be able to give rise to adipocytes, osteoblasts, and chondrocytes under the standard in vitro tissue culture-differentiating conditions.
Sources of mesenchymal stem cells
Mesenchymal stem cells are present in almost all tissues. A significant population of mesenchymal stem cells has been derived from the bone marrow. Cells exhibiting properties of mesenchymal stem cells have also been isolated from adipose tissue, dental tissues, amniotic membrane and fluid, placenta and fetal membrane, endometrium, menstrual blood, peripheral blood, synovial fluid, salivary gland, limb bud, skin and foreskin, sub-amniotic umbilical cord lining membrane and Wharton’s jelly.
Isolation and culture of mesenchymal stem cells
Despite relatively low numbers of MSCs in bone marrow aspirates, there is a keen interest in these cells as they can be easily isolated and expanded in culture through approximately 40 population doublings in 8 – 10 weeks.
Bone marrow is considered to be the best source for mesenchymal stem cells and used as a benchmark for comparison of MSCs obtained from other sources.
Mesenchymal stem cells obtained from bone marrow, peripheral blood and synovial fluid are obtained using Ficoll density gradient method. MSCs obtained from other tissue sources, such as adipose, dental, endometrium, placenta, skin, and foreskin, and Wharton’s Jelly are obtained after digestion with collagenase.
Mesenchymal stem cells isolated from different sources are cultured in Dulbecco’s modified Eagle’s medium (DMEM), DMEM-F12, a-MEM (minimal essential medium), DMEM supplemented with low or high concentration of glucose and RPMI (Rosewell Park Memorial Institute medium). The culture medium was supplemented with either 10% fetal bovine serum (FBS), new-born calf serum (NBCS) or fetal calf serum (FCS).
Expression of cell surface markers
The cells showing positive expression for CD63, D90, and CD105, and lack of expression of CD14, CD34, CD45, and HLA-DR are considered as MSCs. In addition to the above-mentioned markers, MSCs also express CD29, CD44, CD146, and CD140b, depending on the tissue of origin.
Stage-specific embryonic antigen (SSEA)-4, CD146 and stromal precursor antigen-1 (Stro-1) are the hallmarks of mesenchymal stem cells. Stro-1 is positively expressed in bone marrow and dental tissue, but negative in human adipose-derived MSCs.
Capacity for long-term in vitro culturing of mesenchymal stem cells
It is a challenge to obtain an adequate number of cells for clinical applications as they tend to lose their potency during sub-culturing and at higher passages.
Early mesenchymal stem cells show high differentiation potential into chondrocytes, osteocytes, and adipocytes. However, long-term culture and higher passages cause senescence characterized by a decrease in differentiation ability, shortening of telomere length and an increased probability of malignant transformation.
Serum and growth factors impact the properties of mesenchymal stem cells during in vitro culturing. MSCs culturing requires 10% FCS, but MSCs retain FCS proteins that may trigger an immunologic response in vivo.
When mesenchymal stem cells are expanded in serum-free media, there is a gradual decline in differentiation potential and telomerase activity. However, the cells are resistant to malignant transformation and can be expanded at higher passages.
Immunomodulatory effects of mesenchymal stem cells
Mesenchymal stem cells have been shown to suppress the excessive immune response of T and B cells, as well as dendritic cells, macrophages and natural killer (NK) cells by a mechanism that involves the combined effect of many immunosuppressive mediators. Most of the mediators, such as nitric oxide (NO), indoleamine 2,3-dioxygenase (IDO), prostaglandin E2 (PGE2), tumor necrosis factor-inducible gene 6 protein (TSG6), CCL-2, and programmed death ligand 1 (PD-L1) are inducible by inflammatory stimuli.
Although these factors show minimal expression in inactivated mesenchymal stem cells, they can be stimulated by inflammatory cytokines, such as interferon gamma (IFN-g), Tumor necrosis factor alpha (TNF-a) and interleukin -1 (IL-1). MSCs expressing IDO following stimulation with IFN-g catalyze the conversion of tryptophan to kynurenine, which causes the inhibition of the pathway for T-cell proliferation.
Production of NO by mesenchymal stem cells also inhibits T-cell proliferation. MSCs inhibit the maturation of monocytes to dendritic cells leading to reduced T-cell activation. Mesenchymal stem cells also inhibit the upregulation of CD1a, CD40, CD80, and CD86 during DC maturation. Finally, they inhibit the secretion of TNF-a, IFN-g, and IL-12 in dendritic cells and increase the levels of IL-10, inducing a more anti-inflammatory dendritic cell phenotype.
The secretion of soluble factors such as transforming growth factor (TGF-b) and prostaglandin E2 (PGE2) and direct cell-cell contact between MSCs and natural killer (NK) cells suppress the proliferation of NK cells. Cell-cell contact of MSCs through PD-1 binding to its ligand may also be responsible for inhibition of T-cell proliferation.
Sources