To guarantee consumer safety and product quality, biotechnology and pharmaceutical manufacturers should confirm materials across production. The Thermo Scientific™ TruScan™ RM Handheld Raman Analyzer makes use of laboratory-proven Raman spectroscopy to execute precise and trustworthy material identification at the point of need. This helps reduce the requirement for expensive and laborious laboratory sampling tests.
The non-destructive point-and-shoot sampling principle of the portable Raman analyzer streamlines quick verification of a broad range of chemical compounds via sealed packagings such as glass containers, plastic bags, blister packs, and clear gel caps to reduce the threat of contamination and exposure.
TruScan RM Handheld Raman Analyzer technical details
- Compliant with United States Pharmacopeia (USP) Chapter <1120> and European Pharmacopeia (EP) 8.7 general chapter <2.2.48> on Raman spectroscopy
- Fulfills current good manufacturing practices (cGMP) and 21 CFR Part 11 requirements
- Weighs below 2 lbs (0.9 kg)
- Chemical and drop-resistant design
QA/QC applications
- Incoming raw material identification (RMID)
- Finished product inspection
- Dispensing of materials at the time of API manufacture
Brand security application
- Identification of falsified (counterfeit) medicines
Improve productivity and efficiency, minimize lab sampling with the TruScan™ RM Handheld Raman Analyzer
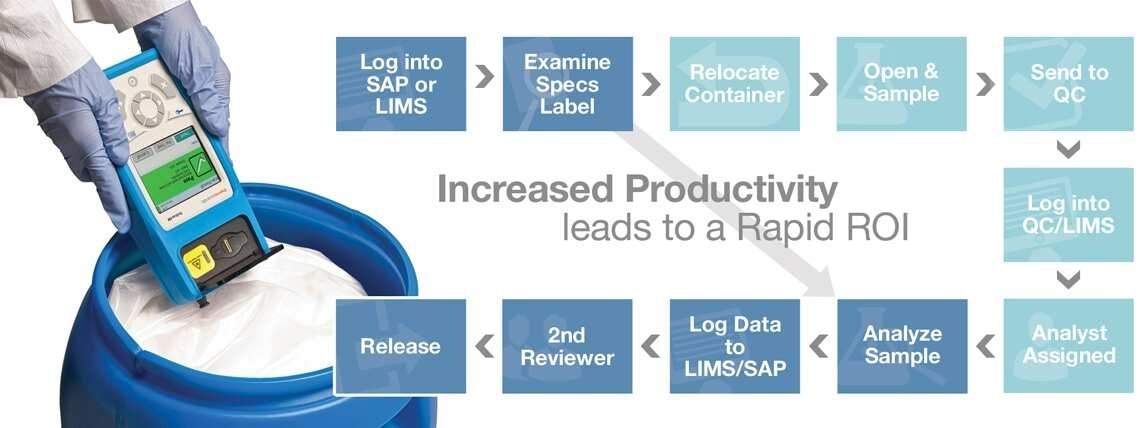
Image Credit: Thermo Fisher Scientific – Handheld Elemental & Radiation Detection
Raman spectroscopy
The technology behind the TruScan RM Handheld Raman Analyzer is Raman spectrometry, a kind of vibrational spectroscopy that specifies materials depending on quantified patterns of wavelengths, or vibrational frequencies.
The analyzer liberates a laser light measuring around 785 nm and helps detect scattered photons at other wavelengths. The scattered light is a special fingerprint for that material, and the TruScan RM Handheld Raman Analyzer uses this “spectral fingerprint” to determine the finished drug or product’s raw material.
The availability of a 785 nm laser wavelength offers an improved balance for quantifying most pharmaceutical materials without compromising Raman signal or intensity spectral resolution.
Patented chemometrics engine
The TruScan RM Handheld Raman Analyzer’s patented multivariate residual analysis provides the highly effective chemometric solution available for material identification—with two spectral pre-processing options—that is simple to operate in sampling conditions and harsh environments.
The TruScan RM Handheld Raman Analyzer obtains the Raman spectrum of the material and, in real-time, identifies the doubt of that measurement, given factors such as the instrument telemetry, sample characteristics, environment, and testing environment.
As the software models the uncertainty (or in standard deviation, statistical terms) directly, there is no user modeling or calibration involved during method development.
TruTools chemometrics capability
Users can surpass simple material ID and determine and measure more materials anywhere in the plant. With the availability of the Thermo Scientific TruTools chemometrics package fixed in the TruScan RM Handheld Raman Analyzer, users could build custom, advanced qualitative and quantitative models for complicated materials analysis issues. Applications include, but are not limited to:
- Finished Product Identification: Differentiate low API–dosage tablets from placebos.
- Raw Material Identification: Guarantee the integrity of users’ inbound raw materials, such as those with the least spectral differences (for example, ethanol vs. methylated spirits).
- Component Threshold Testing: Quantify up to 10 components, then set PASS/FAIL thresholds for fast decision-making (for example, glycerin contaminated with DEG).
- At-line Process Monitoring: Endpoint determination of reaction monitoring, distillations, and component quantification of powder-blended mixtures.
 Image Credit: Thermo Fisher Scientific – Handheld Elemental & Radiation Detection
Image Credit: Thermo Fisher Scientific – Handheld Elemental & Radiation Detection