Identifying Components of a Mixture
Chromatography is a process by which compounds within a mixture are separated. Compounds are be separated by properties such as size, and how the compounds interact with the mobile and stationary phases of chromatography.
The sample is mixed into the mobile phase, usually a liquid or a gas, which is then passed over the stationary phase, usually a solid or a liquid. If a compound (compound A) within a mixture has a low affinity for the stationary phase, then it will not interact much with the stationary phase. However, if another compound (compound B) within a mixture has a high affinity for the stationary phase, then it will bind to the stationary phase. This results in compound A moving through the mobile phase more quickly than compound B, and thus compounds A and B can be separated from the mixture.
Chemistry of thin layer chromatography with plate, solvent and samples. Image Credit: ggw / Shutterstock
Planar vs Column Chromatography
Chromatography techniques can be broadly divided into two types depending on how they are performed: planar and column. In column chromatography, the solid phase is placed within a tube, thus the mobile phase has a 3D space to travel through. Planar chromatography, on the other hand, is when the solid phase is “on a plane”, in 2D.
One advantage of a planar chromatography is that multiple samples can be analyzed at the same time. This means that the conditions of chromatography can be controlled across samples more easily if planar chromatography is used.
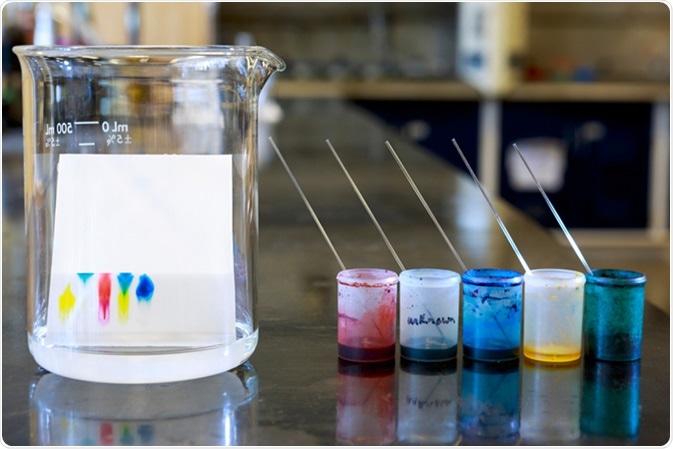
Paper Chromatography
One type of planar chromatography is paper chromatography. Here, the samples are dotted onto a line near one end of a piece of cellulose paper. To run the chromatography, the edge of the paper where the samples have been dotted is then placed into a solvent. The solvent then travels up the cellulose paper, and depending on how the compounds in the sample interact with the stationary (cellulose paper) or mobile (solvent) phases, they travel up the paper at different speeds. This is the “chromatogram” of the samples.
Paper Chromatography Lab
Thin Layer Chromatography
Thin Layer Chromatography is another type of planar chromatography. The stationary phase is usually silica gel or alumina that is placed onto non-reactive material, such as glass. The stationary phase is usually referred to as a “plate”. The samples are dotted onto a line drawn near one end of the plate and dried, like in paper chromatography, and is run like paper chromatography.
Thin-Layer Chromatography (TLC)
How Does Thin Layer Chromatography Work?
The stationary phase of Thin Layer Chromatography is usually silica or alumina, as mentioned above. Silica gel is composed of silicon dioxide (known as silica), where two silicon atoms are joined together by bonding with a shared oxygen atom. However, on the surface of a silica sheet, the silicon atoms are bound to an exposed OH group. This means that the surface of the stationary phase is polar. Alumina is essentially the same as silica, except it is aluminium rather than silicon which is bound to a shared oxygen or an OH group at the surface.
The polar surface means that the stationary phase is able to form hydrogen bonds (via the OH group) with compounds in the mobile phase that are capable of forming these bonds. The polar nature of the Thin Layer Chromatography sheet also means that van der Waals forces and electrostatic interactions occur between the phases too. When a compound is bound by the stationary phase, it is said to be adsorbed.
When the solvent soaks into the Thin Layer Chromatography sheet, it dissolves the sample as it passes over it. The speed at which the compounds within a sample travel up the stationary phase depends on how soluble they are, and also how they react with the stationary phase. For example, if compound A in the sample is able to form hydrogen bonds, it will be adsorbed more strongly than compound B, which cannot form hydrogen bonds. Therefore, compound B will travel up the Thin Layer Chromatography sheet more quickly than compound A, and will result in a higher spot on the chromatogram after a given amount of time.
Viewing the Chromatogram
Some compounds are colorless, so in order to view them on the chromatogram some Thin Layer Chromatography sheets have added compounds which become fluorescent under ultraviolet light (UV), therefore the plate emits light when exposed to UV light. However, the presence of a molecule prevents fluorescence, and is therefore visible as a dark spot.
Certain chemicals can also be used to view compounds. For example, ninhydrin can be sprayed onto the thin layer chromatography sheet, and this interacts with amino acids to give purple or brow spots. Iodine staining can also be used to visualize a Thin Layer Chromatogram by placing the Thin Layer Chromatography plate into a beaker with iodine crystals.
Further Reading