What is Gibbs free energy?
Josiah Willard Gibbs, an American mathematician, first described Gibbs free energy in the 1870s. According to Gibbs, free energy is the total energy of a system that is available to perform useful work. It is expressed in kilojoules (kJ) and is also called “available energy.”
Another widely accepted definition of Gibbs free energy (which is often denoted by the letter G) is that Gibbs free energy measures the maximum work done by a thermodynamic system at constant pressure and temperature.
The free energy is stored in the bonds present in the reactants and products of a reaction. In a thermodynamic reaction, the change in Gibbs free energy (∆G) is represented as:
∆G = total free energy of products – total free energy of reactants
When ∆G = 0, the reaction will be in equilibrium, which means the concentration of products and reactants does not change. ∆G < 0, or a decrease in free energy, means energy is released during the reaction, and when ∆G > 0, or an increase in free energy, it means energy is used up in the reaction.
ATP and free energy
Under standard conditions, glucose reacts with oxygen during glycolysis to release Gibbs free energy. This is an exergonic reaction, which means energy is released. In order to use up this free energy, this reaction is coupled to other reactions in our body. One main way in which this free energy is captured in our body is by producing adenosine triphosphate (ATP) from adenosine diphosphate (ADP) by the addition of an inorganic phosphate group.
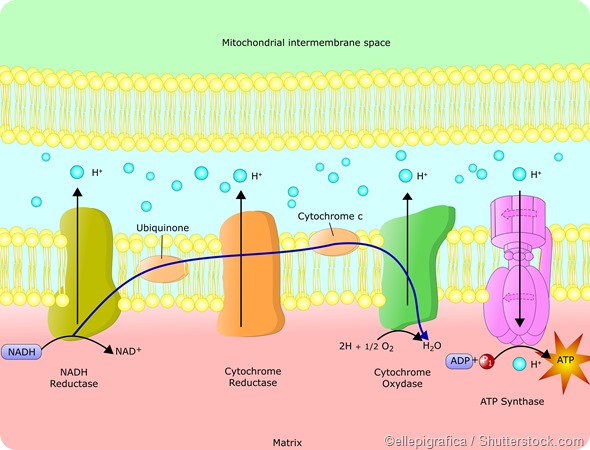
The formation of ATP from ADP is an endergonic reaction, which means energy is used up in the reaction. So by coupling this reaction with the exergonic glycolysis reaction, ATP can be produced.
The oxidation of glucose and the production of ATP in humans results in the storage of large amounts of Gibbs free energy in the phosphate bonds of ATP, which can be released when ATP is hydrolyzed and its phosphate group is removed to form ADP in the cells. This released energy is used up by the body for several cellular processes.
Free energy and metabolism
The breaking down of food particles we consume and derive energy from is called metabolism. It covers various processes that use nutrients in food to release Gibbs free energy, which is stored during the formation of ATP from ADP. This stored energy in ATP is captured and used for various purposes in the body by converting ATP back to ADP.
Nutrient metabolism in the human body occurs via a complex series of enzyme-mediated reactions. According to Hans Krebs, there are three stages in metabolism:
Digestion
The breaking down of large biomolecules such as carbohydrates, lipids, and proteins to less complex, smaller molecules such as glucose, fatty acids, and amino acids, respectively. These simpler molecules can easily travel from the digestive tract and enter the bloodstream.
Conversion to acetyl CoA
The smaller molecules of glucose, fatty acids, and amino acids are converted to acetyl groups which get linked to coenzyme A forming acetyl coenzyme A, or acetyl CoA.
Oxidation of acetyl groups
The acetyl CoA is oxidized during the citric acid cycle or Krebs cycle. This oxidation reaction, which converts acetyl CoA to CO2 and H2O, is coupled with other reactions that produce ATP from ADP by a process called oxidative phosphorylation.
Thus, ATP production in cells stores Gibbs free energy which can then be used for other processes with the help of the reverse reaction which converts ATP to ADP and, in the process, releases 30.5 kJ of energy per ATP molecule.
Gibbs free energy introduction | Biomolecules | MCAT | Khan Academy
References
- http://www.ncbi.nlm.nih.gov/books/NBK9903/
- http://ocw.mit.edu/courses/biology/7-014-introductory-biology-spring-2005/recitations/section4_ak.pdf
- https://healthyfitnessai.com/en/l
Further Reading