Cellular immunotherapy—for example, Chimeric Antigen Receptor (CAR)-T cell therapy—has shown great efficacy in treating various hematologic malignancies. CAR-T therapy has undergone notable changes, and several drugs have received regulatory approval for commercial use.
Despite these achievements, substantial challenges remain in the widespread use of autologous CAR-T therapy, such as the complexities of obtaining patient T cells, lengthy manufacturing processes, and complications in scale-up manufacturing procedures. These challenges must be addressed.
Efficient CAR-T therapy production often relies on securing a robust supply of the patient's T cells. To tackle this challenge, scientists have identified alternative sources for T cells, such as embryonic stem cells (ESCs) and, notably, induced pluripotent stem cells (iPSCs).
iPSCs have recently emerged as a promising solution, thanks to their unique self-renewal properties and their potential for gene editing. iPSCs provide a renewable reservoir of T cells, establishing a platform for cell therapy with an "infinite supply."
The strategic use of iPSCs addresses crucial bottlenecks in traditional methods, representing significant progression in cellular immunotherapy.
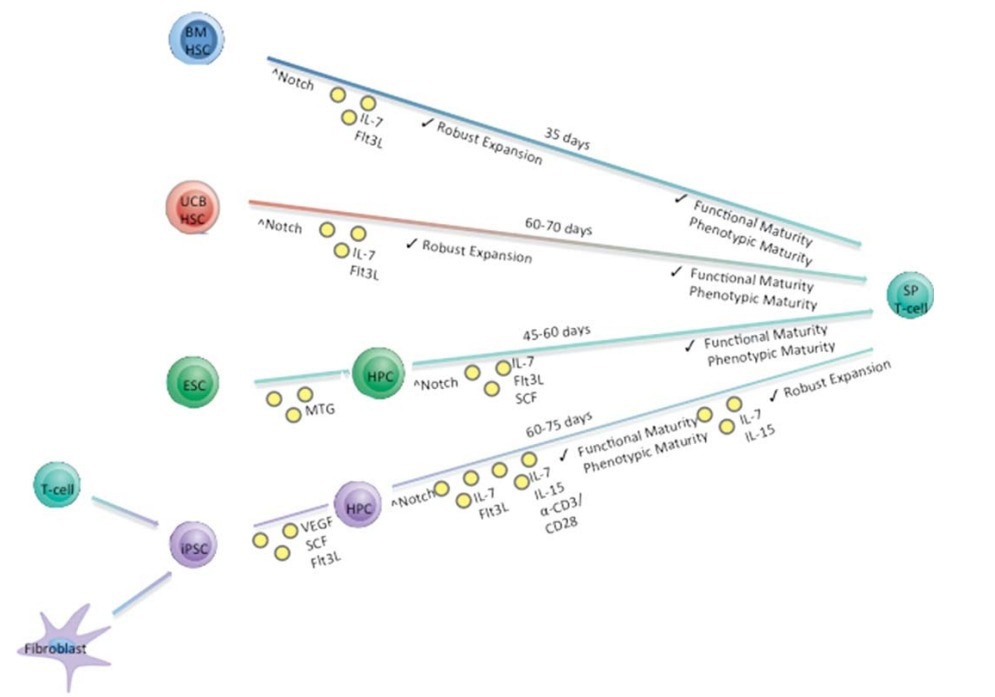
Simplified in vitro differentiation schema describing the approximate time needed to generate mature SP T cells from four starting progenitor cell populations. Stem Cells. 2015;33(11):3174-3180.1 Image Credit: ACROBiosystems
Delta-like protein 4 (DLL4) plays a critical role in iPSC differentiation into T cells
The traditional method to differentiate iPSCs into T cells involves co-cultivating iPSCs obtained from a healthy donor with murine bone marrow-derived stromal cell lines, specifically OP9. This process enables the creation of CD34+ hematopoietic progenitor cells (HPCs).
The HPCs then undergo enrichment using specific growth factors (SCF, Flt3, IL-7, IL-3, and TPO), in conjunction with co-cultivation with OP9 cells that either overexpress DLL1 or DLL4. This effectively triggers the T-cell differentiation, resulting in the successful production of iPSC-induced differentiated T cells.
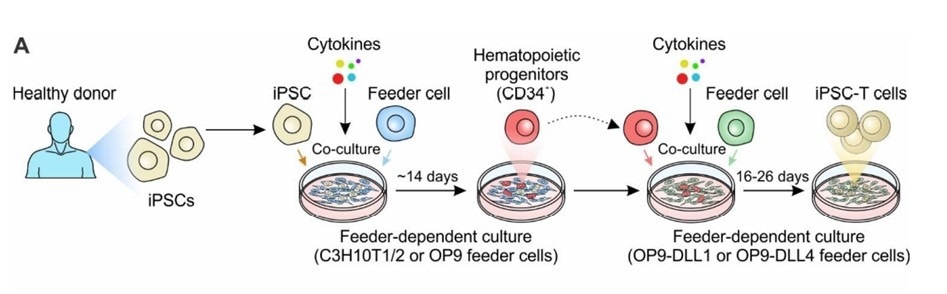
Generation of iPSC-derived T cell-based cell therapy. Cancers (Basel). 2022;14(9):2266.2 Image Credit: ACROBiosystems
The physical interaction between Notch receptors and their ligands is essential in developing various cell lineages extending beyond T cells. In generating T cells from iPSCs, this interaction is primarily coordinated by Notch1 and DLL4.
The interplay occurs by connecting the Notch1 receptor (on HPCs) and DLL1/DLL4 (present on OP9-DL cells). This intercellular communication triggers a series of precisely regulated steps, ultimately leading to the differentiation of iPSCs into the T cell lineage.
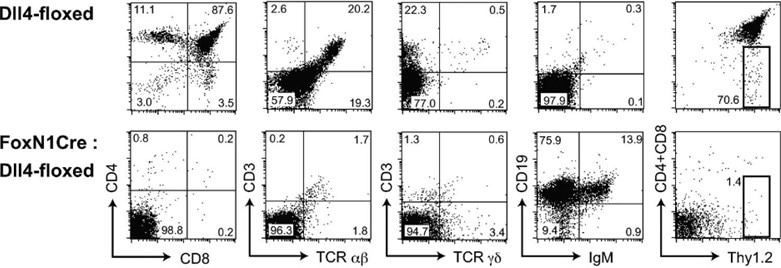
No T cells, but aberrant B cell accumulation, in the thymus with Dll4-null epithelial cells, J Exp Med. 2008;205(11):2507-2513.3 Image Credit: ACROBiosystems
DLL1 and DLL4 have proven their capability to support T cell-derived iPSCs, iPSC-T.
To further explore the comparative effectiveness of these DLL variants in inducing HPCs into the T cell lineage in vitro, a series of OP9 cells expressing either DLL1 or DLL4 with varying expression levels (low, medium, and high) were employed to clarify the induction of HPCs into T cells.
Surprisingly, the findings revealed that despite the lower cell surface protein level of DLL4-HA on OP9-DL4hi and OP9-DL4med cells compared to DLL1-HA on OP9-DL1hi and OP9-DL1med cells, feeder cells expressing DLL4 demonstrated higher efficacy in supporting T cell generation.
To summarize, this research presents DLL4 as a more efficient inducer of HPCs in the T cell lineage when compared to DLL1.
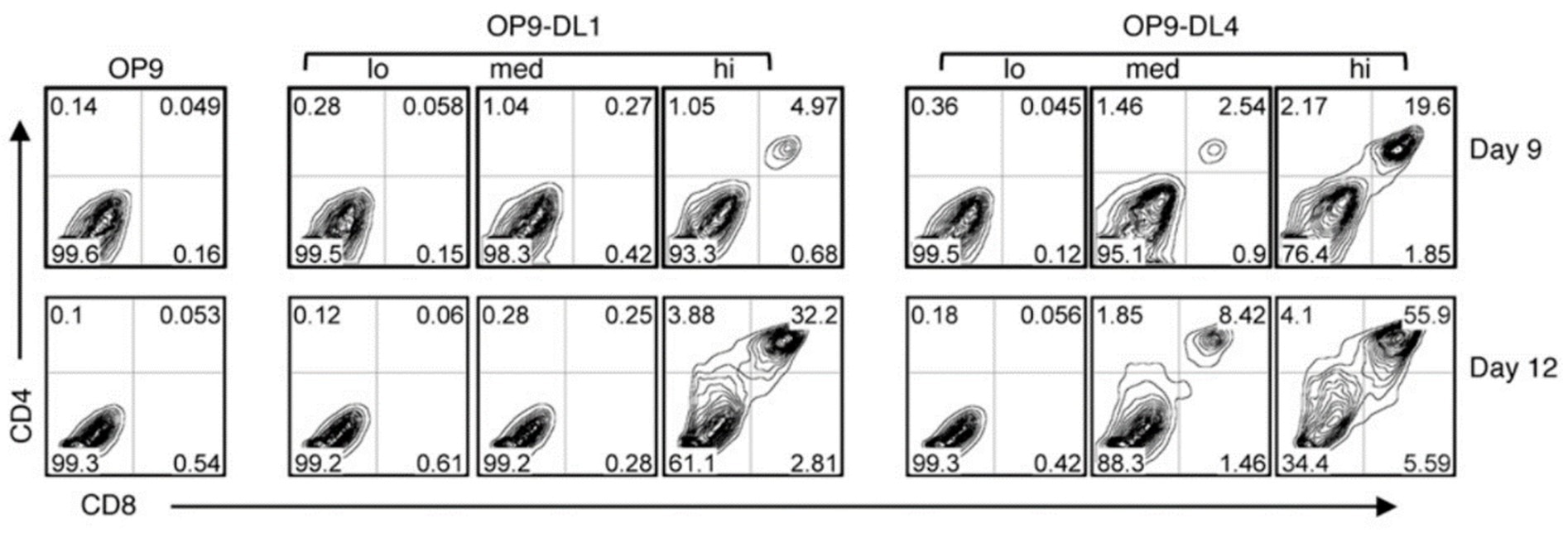
Comparison of T cell development from HPCs cultured with OP9-DL1 (lo/med/hi) and OP9-DL4 (lo/med/hi) cell lines. J Immunol. 2010;185(2):867-876.4 Image Credit: ACROBiosystems
In the traditional approach, feeder cells such as OP9 play a crucial role in the induction and differentiation of iPSC-T. However, relying on mouse-derived stromal cell lines and certain unspecified extracts poses the risk of cross-species contamination, creating challenges for rigorous quality control in clinical applications.
To address these concerns, feeder-cell-free cultures utilizing recombinant DLL4 protein have become the leading solution for producing clinical-grade iPSC-T. This method involves plates combined with DLL4 and adhesion molecules like VCAM-1, enhancing clinical translational potential.
Notably, the Zúñiga-Pflücker group at the University of Toronto has advanced the field by developing feeder-cell-free in vitro systems that utilize DLL4 microbeads, which substantially improves support for large-scale suspension culture of iPSCs.5
T cell development from human pluripotent stem cells using DLL4-μbeads, Nat Commun. 2021;12(1):5023.5 Image Credit: ACROBiosystems
Using GMP-grade DLL4 to replace feeder cells
As iPSCs become a promising and versatile cell source for CAR-T therapy, expanding the scale of cellular differentiation into iPSC-T cells is a crucial step in overcoming manufacturing challenges faced by current CAR-T therapies.
Despite the common use of feeder cells for differentiation, they pose a significant risk to the safety of biologics, particularly during scale-up and clinical applications.
With the introduction of GMP-grade DLL4, adding individual recombinant factors can replace feeder cells, with recent research achieving sufficient numbers for safety evaluations in clinical trials. This is a key advancement in addressing challenges associated with autologous cell therapy.
The availability of GMP-grade DLL4 tackles issues related to T-cell scarcity in patients and expands the potential beneficiaries in cell therapy.
In the ever-evolving landscape of cancer treatment, where cellular immunotherapy takes center stage, the advancement in iPSC-T cell manufacturing has immense potential to support a new era of therapeutic possibilities.
ACROBiosystems is committed to developing top-notch reagents and raw materials for CGT clinical stages. With a robust GMP quality management system, ACROBiosystems is delighted to announce the launch of its GMP-grade recombinant DLL4 protein, showcasing exceptional activity and safety.
This protein is well-suited for supporting the feeder-cell-free approach to iPSC-to-T cell differentiation, especially in large-scale clinical manufacturing of iPSCs.
References and further reading
- Simplified in vitro differentiation schema describing the approximate time needed to generate mature SP T cells from four starting progenitor cell populations. Stem Cells. 33(11):3174-3180 (2015)
- Yang Zhou, Miao Li, Kuangyi Zhou, James Brown, Tasha Tsao, Xinjian Cen, Tiffany Husman, Aarushi Bajpai, Zachary Spencer Dunn, and Lili Yang. Engineering Induced Pluripotent Stem Cells for Cancer Immunotherapy. Cancers (Basel). 14(9): 2266 (2022).
- Katsuto Hozumi, Carolina Mailhos, Naoko Negishi, Ken-ichi Hirano, Takashi Yahata, Kiyoshi Ando, Saulius Zuklys, Georg A. Holländer, David T. Shima, Sonoko Habu. Delta-like 4 is indispensable in thymic environment specific for T cell development. J Exp Med. 205 (11): 2507–2513(2008)
- Mahmood Mohtashami; Divya K. Shah; Hiroshi Nakase; Korosh Kianizad; Howard T. Petrie; Juan Carlos Zúñiga-Pflücker. Direct Comparison of Dll1- and Dll4-Mediated Notch Activation Levels Shows Differential Lymphomyeloid Lineage Commitment Outcomes. J Immunol.185 (2): 867–876 (2010).
- Trotman-Grant A.C., Mohtashami M., De Sousa Casal J., Martinez E.C., Lee D., Teichman S., Brauer P.M., Han J., Anderson M.K., Zuniga-Pflucker J.C. DL4-mubeads induce T cell lineage differentiation from stem cells in a stromal cell-free system. Nat. Commun. 12:5023. (2021).
About ACROBiosystems
ACROBiosystems is a leading manufacturer of recombinant proteins and other critical reagents to support the development of target therapeutics, vaccines, and diagnostics. The company employs an application-oriented development strategy, with a particular focus on product design, quality control, and solution-based support. Our products and services enable anyone in the field of drug development to have a more intuitive and streamlined process.
ACROBiosystems' catalog includes a comprehensive list of disease-associated biomarkers and drug targets from humans to other common species. All of our products are produced with high quality and batch-to-batch consistency to satisfy the rigorous standards of pharmaceutical research and development.
ACROBiosystems continue to grow and adapt to bring more value to our clients by providing more technical resources and custom services, promoting training and communication opportunities, initiating collaborations in the bio-industry community, and facilitating transactions in the dynamic global and each niche market.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.