Enzyme kinetics involves the measurement of the rate at which chemical reactions that are catalyzed by enzymes occur. Knowledge about the kinetics of an enzyme can reveal useful information about its catalytic mechanism, role in metabolism, factors that impact its activity, and mechanisms of inhibition.
This article will cover the basic principles of enzyme kinetics, including the reaction equation, rate of reaction and maximal velocity (Vmax) and Michaelis Constant (Km).
Rate of reaction
Enzymes are thought to form a complex with the substrates to catalyze the reaction. This process can be illustrated with the simplified equation, where e is the enzyme, S is the substrate, and P is the product:
E + S ⇔ ES ⇒ P + E
The first step of the equation, which is reversible, has the reaction rate constant of k+1 to produce the enzyme substrate complex and k-1 for the reverse reaction. The reaction rate constant for the second step of the equation, which is not reversible, is k+2.
The rate of reaction (v), which is the rate at which the product is formed, is defined by the following equation:
v = d[P]/dt = k+2[ES]
The square brackets in the above equation represent the molar concentration of the substance specified within, so [P] refers to the molar concentration of the product and [ES] the molar concentration of the enzyme substrate.
Vmax and Km
Leonor Michaelis and Maud Menten introduced a mathematical illustration to describe the action of enzymes with two constants, Vmax and Km.
The maximal velocity (Vmax) refers to the point at which the increase the concentration of the substrate does not increase the rate of a reaction catalyzed by an enzyme. This occurs because the substrate molecules saturate the active sites of the enzyme and are not able to form more complexes with the enzyme. This value is given as a rate (mmol/s), which is the maximum velocity of the reaction when the enzyme is saturated.
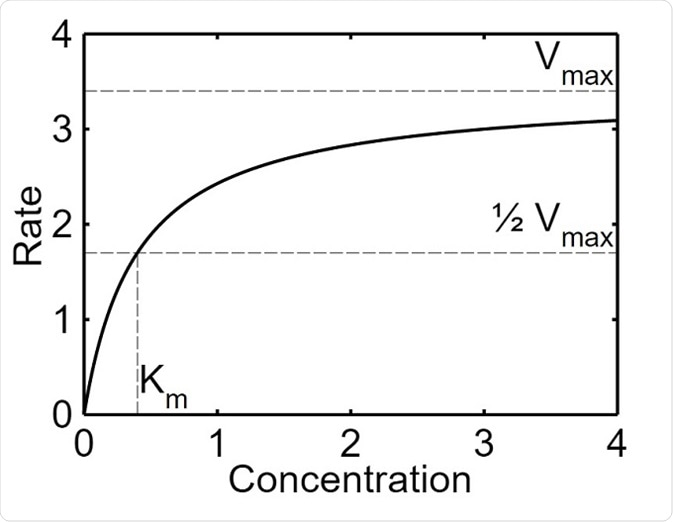
Michaelis–Menten saturation curve of an enzyme reaction. Parameter values used are Vmax=3.4 and Km=1.7.
The Michaelis constant (Km) is the concentration of the substrate when half of the active binding sites of an enzyme are occupied by the substrate. The constant helps to depict the affinity of the enzyme for their substrate. This value is given as the concentration of the substrate (mM) at half of Vmax. An enzyme with a high Km has a low affinity for the substrate, and a high concentration of the substrate is needed in order for the enzyme to become saturated. Conversely, an enzyme with a high Km has a high affinity for the substrate and the enzyme may become saturated even with a low amount of substrate.
Applications of enzyme kinetics
There are many practical uses of enzyme kinetics. For example, the kinetic constants can help explain how enzymes work and assist in the prediction of the behavior of enzymes in living organisms.
Vmax and Km both play a key role in understanding the metabolism of the human body. Knowledge of the enzyme kinetic constants allows us to gain a better understanding of the enzymes and processes that take place in human metabolism. This knowledge could then be used for medical purposes to improve patient health outcomes.
References
Further Reading