Adoptive cell therapy is a promising alternative option for treating both solid and hematological cancers, with CAR-T therapy standing out as a prominent approach. In this method, T cells undergo genetic modification with chimeric antigen receptors (CARs) to improve their targeting abilities.1-2
The effectiveness of CAR-T cell treatment relies on the intricate interplay of phenotype, activation, and functional profiling of these modified cells. Immunophenotypic analysis of CAR-T cells plays a crucial role in guaranteeing treatment quality and enabling continual monitoring of treatment response.1
During the immunophenotyping process, engineered T cells are segregated according to their markers to characterize the composition of the cell population in the specimen. The deliberate identification and separation of specific subsets of CAR-T cells is vital for enhancing treatment responses.2
This article describes the features and role of CAR-T therapy in the immunophenotyping and treatment of solid cancers.
Deciphering cellular composition, defining CAR-T therapy efficacy
Immunophenotyping is an important method that couples specific antibodies with fluorescent compounds to uncover protein expression in cell populations to detect and categorize the tagged cells.
This technique exploits variations in surface markers among T cells, reflecting differences in their activation, differentiation, and memory status.2
These markers offer insights into immune cell development, function, proliferation potential, and long-term viability. The specific surface marker profiles correlate with the effectiveness of CAR-T cell therapy.3
Table 1 presents key markers for immunophenotypic analysis, including CD3, CD4, CD8, CD45RA, CD34R0, CCR7, CD27, and CD95.
Table 1. A comprehensive phenotype of markers of T cell subsets (DOI: 10.1007/978-1-0716-0146-4). Source: Sino Biological Inc.
| Cell type |
CD3 |
CD4 |
CD8 |
CD45RA |
CD45RO |
CCR7 |
CD27 |
CD95 |
CAR |
| Naive T cell |
+
+ |
+
|
+ |
+
+ |
-
- |
|
+
+ |
|
|
| Naive CAR-T cell |
+
+ |
+
|
+ |
+
+ |
-
- |
|
+
+ |
|
+
+ |
| Central memory T cell |
+
+ |
+
|
+ |
-
- |
+
+ |
|
+
+ |
|
|
| Central memory CAR-T cell |
+
+ |
+
|
+ |
-
- |
+
+ |
|
+
+ |
|
+
+ |
| Effector memory T cell |
+
+ |
+
|
+ |
-
- |
+
+ |
|
-
- |
|
|
| Effector memory CAR-T cell |
+
+ |
+
|
+ |
-
- |
+
+ |
|
-
- |
|
+
+ |
Effector
T cell |
+
+ |
+
|
+ |
-
- |
-
- |
|
-
- |
|
|
Effector
CAR-T cell |
+
+ |
+
|
+ |
-
- |
-
- |
|
-
- |
|
+
+ |
| Stem cell memory-like T cell |
+
+ |
+
|
+ |
|
-
- |
+
+ |
+
+ |
+
+ |
|
| Stem cell memory-like CAR-T cell |
+
+ |
+
|
+ |
|
-
|
+
+ |
+
+ |
+
+ |
+
+ |
Flow cytometry (FCM) is the preferred approach for assessing the immunophenotype and functionality of CAR-T cell subsets.4 The success of experiments is directly influenced by the quality of Flow cytometry (FACS) antibodies.
FCM also allows for the monitoring of CAR-T cells in a patient's blood, which is crucial for predicting the probability and severity of cytokine release syndrome (CRS) or neurotoxicity.5
To aid research on immunophenotyping, Sino Biological has established a comprehensive collection of recombinant T cell biomarkers and T cell-related FACS exhibiting high affinity and specificity, including CD3, CD4, CD8, and CD45.
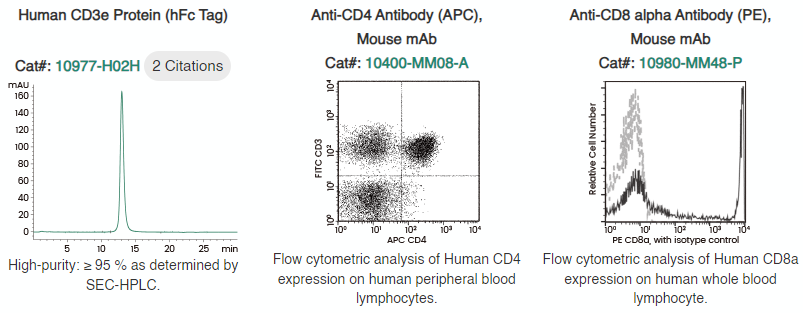
Figure 1. Quality products for T cell markers offered by Sino Biological. Image Credit: Sino Biological Inc.
Selecting T cell subsets, optimizing CAR-T therapy efficacy
Enhancing the effectiveness of CAR-T therapy requires the pre-selection of T cell populations before CAR engineering. T lymphocytes can be immunophenotyped based on CD3 expression.
This can be further categorized into CD4+ and CD8+ T cells, which are distinguished by their subsets and their effector and memory functions. They demonstrate various characteristics regarding internal and external markets, epigenetic factors, genetic programs, and metabolic pathways.6
These subsets are intrinsically tied to CD3 molecule interactions in the T cell receptor (TCR) signaling pathway. They significantly impact CAR-T cell phenotype and therapeutic efficacy.5-7
Certain studies suggest that CD4+ CAR-T cells may trigger more powerful antitumor responses against specific solid tumors when compared to CD8+ cells.8
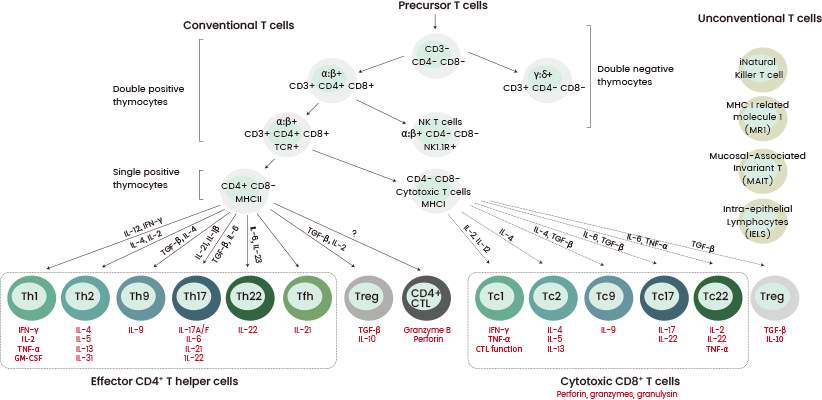
Figure 2. Schematic overview of the T cell subsets https://doi.org/10.1111/all.15104. Image Credit: Sino Biological Inc.
From the standpoint of the activation phase, Naive and memory T cells have prolonged persistence and heightened antitumor activity, and thus surpass effector T cells in therapeutic potential.9
CAR-T cells that display a memory-like phenotype, identified by surface markers like CD62L, CD45RA, CCR7, and CD45RO, exhibit superior efficacy.3
Exhaustion-like phenotypes, on the other hand, are characterized by the expression of inhibitory receptors (PD-1, LAG3, TIM-3, CTLA4, and TIGIT) and exhibit limited therapeutic potency.3,10
CAR-T cell products derived from preselected naive/stem memory T cells (TN/SCM) show greater expansion potential and a smaller risk of cytokine release syndrome (CRS).11
Unveiling activation and exhaustion states, ensuring CAR-T therapy efficacy
T cell activation plays a crucial role in CAR-T cell production, influencing transduction efficiency, cell expansion rate, and differentiation. The expression of surface receptors, including CD69, CD25, CD71, and MHC-II, assists in assessing T cell activation phases.12
Co-stimulatory molecules like CD26, CD27, CD28, CD30, CD154, or CD40L may additionally be expressed upon activation.13
Activated T cells can induce toxicities through the release of inflammatory cytokines such as TNF-α and IFN-γ.14-15 Monitoring these cytokine concentrations ensures the effectiveness of the T cell population (Figure 3). Tailoring the dosage of T cell stimulation affects CAR-T cell functionality, resulting in a more consistent and potent therapeutic product.16-17
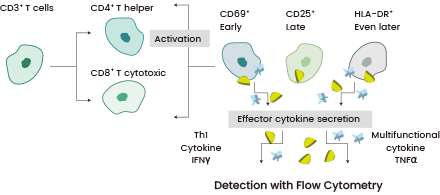
Figure 3. Different T cell phenotypes are profiled for the expression of 3 activation markers: CD69 (early), CD25 (late), and HLA-DR (even later). The 2 effector cytokines (IFNγ and TNFα) are also quantified using sandwich ELISA. Image Credit: Sino Biological Inc.
The performance of CAR-T cells is regulated by cell exhaustion, which reduces their persistence and killing activity, resulting in a diminished therapeutic potential against solid tumors.
Exhaustion markers such as PD-1, LAG3, TIM-3, CTLA4, and TIGIT provide insights into the state of CAR-T cell exhaustion, underscoring the importance of early T cell utilization to mitigate exhaustion-related limitations.18 The selection of different cytokine cocktails or suitable cytokines for CAR-T cell culture can significantly enhance the efficacy of CAR-T cells.
Sino Biological has successfully developed an extensive range of high-quality GMP-grade cytokines, along with ELISA kits designed for the detection and measurement of residual cytokines. Additionally, Sino Biological offers a panel of recombinant exhaustion marker proteins with high purity and bioactivity.
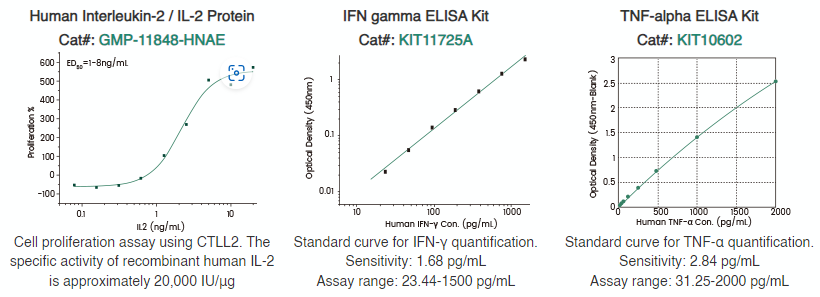
Figure 4. Premium quality cytokines and ELISA kits. Image Credit: Sino Biological Inc.
Sino biological's support for CAR-T therapy and immunophenotyping
Immunophenotyping shows significant potential in enhancing CAR-T cell therapy by improving therapeutic efficiency and predicting treatment responses.
Sino Biological, a globally leading supplier of bioreagents and CRO services, provides comprehensive solutions for CAR-T cell therapy. These solutions encompass reagents and services that assist clients throughout each stage, from early target discovery to the preclinical phase of research and development.
Sino Biological offers a diverse range of reagents for the development of CAR-T cell therapy, featuring over 250 CAR-T target proteins, flow cytometry antibodies, GMP-grade cytokines, and GMP-grade SuperNuclease.
Stringent quality control measures are implemented to ensure the efficacy and safety of CAR-T cell products. These measures include assessments of cell viability, CAR detection, population profiling, and cytotoxicity.
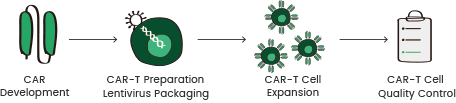
Figure 5. Comprehensive solutions for CAR-T therapies provided by Sino Biological. Image Credit: Sino Biological Inc.
References and further reading
- Majzner, R. G., & Mackall, C. L. (2019). Clinical lessons learned from the first leg of the CAR T cell journey. Nature medicine, 25(9), 1341–1355. https://doi.org/10.1038/s41591-019-0564-6
- Chimeric antigen receptor T cells. Springer Science+Business Media, 2020. doi: 10.1007/978-1-0716-0146-4.
- Si, X., Xiao, L., Brown, C. E., & Wang, D. (2022). Preclinical Evaluation of CAR T Cell Function: In Vitro and In Vivo Models. International journal of molecular sciences, 23(6), 3154. https://doi.org/10.3390/ijms23063154
- U. Blache et al., “Advanced flow cytometry assays for immune monitoring of CAR-T cell applications,” Frontiers in Immunology, vol. 12, May 2021, doi: 10.3389/fimmu.2021.658314.
- Demaret, J., Varlet, P., Trauet, J., Beauvais, D., Grossemy, A., Hégo, F., Yakoub-Agha, I., & Labalette, M. (2021). Monitoring CAR T-cells using flow cytometry. Cytometry. Part B, Clinical cytometry, 100(2), 218–224. https://doi.org/10.1002/cyto.b.21941
- Golubovskaya, V., & Wu, L. (2016). Different Subsets of T Cells, Memory, Effector Functions, and CAR-T Immunotherapy. Cancers, 8(3), 36. https://doi.org/10.3390/cancers8030036
- M. E. Call and K. W. Wucherpfennig, “Molecular mechanisms for the assembly of the T cell receptor–CD3 complex,” Molecular Immunology, vol. 40, no. 18, pp. 1295–1305, Apr. 2004, doi: 10.1016/j.molimm.2003.11.017.
- D. Kravtsov, A. K. Erbe, P. M. Sondel, and A. L. Rakhmilevich, “Roles of CD4+ T cells as mediators of antitumor immunity,” Frontiers in Immunology, vol. 13, Sep. 2022, doi: 10.3389/fimmu.2022.972021.
- López-Cantillo, G., Urueña, C., Camacho, B. A., & Ramírez-Segura, C. (2022). CAR-T Cell Performance: How to Improve Their Persistence?. Frontiers in immunology, 13, 878209. https://doi.org/10.3389/fimmu.2022.878209
- 10) C. R. F. Silveira et al., “Cytokines as an important player in the context of CAR-T cell therapy for cancer: Their role in tumor immunomodulation, manufacture, and clinical implications,” Frontiers in Immunology, vol. 13, Sep. 2022, doi: 10.3389/fimmu.2022.947648.
- S. Arcangeli et al., “CAR T cell manufacturing from naive/stem memory T lymphocytes enhances antitumor responses while curtailing cytokine release syndrome,” Journal of Clinical Investigation, vol. 132, no. 12, Jun. 2022, doi: 10.1172/jci150807.
- M. M. Naghizadeh, N. Hatamzade, F. G. Larsen, R. B. Kjærup, E. Wattrang, and T. S. Dalgaard, “Kinetics of activation marker expression after in vitro polyclonal stimulation of chicken peripheral T cells,” Cytometry Part A, vol. 101, no. 1, pp. 45–56, Jan. 2021, doi: 10.1002/cyto.a.24304.
- M. Shipkova and E. Wieland, “Surface markers of lymphocyte activation and markers of cell proliferation,” Clinica Chimica Acta, vol. 413, no. 17–18, pp. 1338–1349, Sep. 2012, doi: 10.1016/j.cca.2011.11.006.
- M. E. Martinez-Sanchez, L. Huerta, E. R. Alvarez-Buylla, and C. V. Lujan, “Role of cytokine combinations on CD4+ T cell differentiation, partial polarization, and plasticity: Continuous Network Modeling approach,” Frontiers in Physiology, vol. 9, Aug. 2018, doi: 10.3389/fphys.2018.00877.
- J. Zhu and W. E. Paul, “CD4 T cells: fates, functions, and faults,” Blood, vol. 112, no. 5, pp. 1557–1569, Sep. 2008, doi: 10.1182/blood-2008-05-078154.
- Y. Lee et al., “Regulation of CAR T cell-mediated cytokine release syndrome-like toxicity using low molecular weight adapters,” Nature Communications, vol. 10, no. 1, Jun. 2019, doi: 10.1038/s41467-019-10565-7.
- N. Lapteva et al., “T-Cell receptor stimulation enhances the expansion and function of CD19 Chimeric Antigen Receptor–Expressing T cells,” Clinical Cancer Research, vol. 25, no. 24, pp. 7340–7350, Dec. 2019, doi: 10.1158/1078-0432.ccr-18-3199.
- Kouro, T., Himuro, H. & Sasada, T. Exhaustion of CAR T cells: potential causes and solutions. J Transl Med 20, 239 (2022). https://doi.org/10.1186/s12967-022-03442-3
About Sino Biological Inc.
Sino Biological is an international reagent supplier and service provider. The company specializes in recombinant protein production and antibody development. All of Sino Biological's products are independently developed and produced, including recombinant proteins, antibodies, and cDNA clones. Sino Biological is the researchers' one-stop technical services shop for the advanced technology platforms they need to make advancements. In addition, Sino Biological offers pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates.
Sino Biological's core business
Sino Biological is committed to providing high-quality recombinant protein and antibody reagents and to being a one-stop technical services shop for life science researchers around the world. All of our products are independently developed and produced. In addition, we offer pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates. Our product quality control indicators meet rigorous requirements for clinical use samples. It takes only a few weeks for us to produce 1 to 30 grams of purified monoclonal antibody from gene sequencing.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.