Atrovent 250 µg/1 mL and Atrovent Adult 500 µg/1 mL Unit Dose Vial (UDV)
ipratropium bromide monohydrate
Consumer Medicine Information
What is in this leaflet
This leaflet answers some common questions about Atrovent UDV.
It does not contain all the available information.
It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor or pharmacist has weighed the risks
of you using Atrovent UDV against the benefits it is expected to have for you.
If you have any concerns about using this medicine, ask your doctor or pharmacist.
This leaflet was last updated on the date at the end of this leaflet. More recent
information may be available. The latest Consumer Medicine Information is available
from your pharmacist, doctor, or from
www.medicines.org.au and may contain important information about the medicine and its use of which you
should be aware.
Keep this leaflet with your medicine.
You may need to read it again.
What Atrovent UDV is used for
Atrovent UDV is used to treat:
asthma
chronic obstructive bronchitis
people who have difficulty breathing during or after surgery using assisted ventilation.
Asthma is a disease where the lining of the lungs becomes inflamed (red and swollen),
making it difficult to breathe. This may be due to an allergy to house dust mites,
smoke or other irritants.
Chronic obstructive bronchitis is a lung condition that can cause difficulty in breathing,
wheeziness and constant coughing.
Atrovent UDV contains the active ingredient ipratropium bromide monohydrate. It belongs
to a group of medicines called anticholinergic bronchodilators.
Atrovent UDV opens up the air passages in people suffering from asthma, chronic bronchitis
and difficulty breathing during or after surgery.
It begins to act quickly after use but may take up to 2 hours to give maximum benefit.
Ask your doctor if you have any questions about why Atrovent UDV has been prescribed
for you.
Your doctor may have prescribed Atrovent UDV for another reason.
There is no evidence that Atrovent UDV is addictive.
Atrovent UDV is available only with a doctor’s prescription.
Before you use Atrovent UDV
When you must not use it
Do not use Atrovent UDV if you have an allergy to:
ipratropium bromide monohydrate or any other medicines used to treat breathing problems
similar medicines which contain atropine or medicines like atropine
any of the ingredients listed at the end of this leaflet
any other anticholinergic medicines.
Some of the symptoms of an allergic reaction may include:
shortness of breath, wheezing or troubled breathing
swelling of the face, lips, tongue or other parts of the body
rash, itching or hives on the skin.
Do not use Atrovent UDV after the expiry date printed on the pack or if the packaging
is torn or shows signs of tampering.
If it has expired or is damaged, return it to your pharmacist for disposal.
If you are not sure whether you should start using Atrovent UDV, contact your doctor
or pharmacist.
Before you start to use it
Tell your doctor or pharmacist if you have allergies to any other medicines, foods,
preservatives or dyes.
Tell your doctor or pharmacist if you are pregnant or intend to become pregnant.
Your doctor or pharmacist will discuss the possible risks and benefits of using Atrovent UDV
during pregnancy.
Tell your doctor or pharmacist if you are breast-feeding or plan to breast-feed.
Your doctor or pharmacist will discuss the possible risks and benefits of using Atrovent
UDV during breast-feeding.
Tell your doctor or pharmacist if you have or have had any of the following medical
conditions:
glaucoma (high pressure in the eye)
difficulty or pain when passing urine
constipation
cystic fibrosis
hyperreactive airway.
If you have not told your doctor or pharmacist about any of the above, tell them before
you start using Atrovent UDV.
Using other medicines
Tell your doctor if you are taking any other medicines, including any that you buy
without a prescription from your pharmacy, supermarket or health food shop.
Some medicines and Atrovent UDV may interfere with each other. These include:
medicines used to treat heart problems such as adrenaline
medicines used to treat asthma or a lung condition called chronic obstructive pulmonary
disease (COPD) such as theophylline, salbutamol or tiotropium
other nebuliser solutions such as disodium cromoglycate.
This is because some nebuliser solutions may not mix well with Atrovent UDV and may
need to be nebulised separately.
These medicines may be affected by Atrovent UDV, or may affect how well it works.
You may need different amounts of your medicines, or you may need to take different
medicines.
Atrovent UDV may be used with other medicines that relax the air passages for additional
symptom relief, when prescribed by a doctor.
Your doctor and pharmacist may have more information on medicines to be careful with
or avoid while using Atrovent UDV.
How to use Atrovent UDV
You will find instructions on how to use Atrovent UDV at the end of this leaflet.
Follow all directions given to you by your doctor or pharmacist carefully.
They may differ from the information contained in this leaflet.
If you are not sure how to use a nebuliser mask, or do not understand the instructions
on the box, ask your doctor or pharmacist for help.
Children should only use Atrovent 250 µg/1mL UDV on medical advice and with the help
of an adult.
Fit the nebuliser mask to your or your child’s nose and mouth before nebulising and
inhaling to prevent the mist from contacting your eyes. If you find it difficult to
breathe in and use your nebulising mask at the same time, talk to your doctor or pharmacist.
They may be able to recommend another method.
How much to use
The usual doses for adults and children are stated below.
Adults:
1 to 2 vials (250 µg to 500 µg) of Atrovent 250 µg/1mL UDV, or
1 vial of Atrovent Adult 500 µg/mL UDV
diluted to 2-3 mL with normal saline and nebulised until the entire volume of solution
is inhaled, 4 times daily.
Children:
1 vial of Atrovent 250 µg/1mL UDV, diluted to 2-3 mL with normal saline and nebulised
until the entire volume of solution is inhaled, 4 times daily.
Depending on your condition and specific needs, your doctor may advise you to take
a different dose.
When to use it
Use your medicine at about the same time each day.
Using it at the same time each day will have the best effect. It will also help you
remember when to use it.
If you forget to use it
If it is almost time for your next dose, skip the dose you missed and have your next
dose when you are meant to.
Otherwise, use it as soon as you remember, and then go back to using your medicine
as you would normally.
Do not use a double dose to make up for the dose that you missed.
This may increase the chance of you getting an unwanted side effect.
If you are not sure what to do, ask your doctor or pharmacist.
If you have trouble remembering to use your medicine, ask your pharmacist for some
hints.
How long to use it
Continue using Atrovent UDV for as long as your doctor tells you.
Atrovent UDV helps control your condition, but does not cure it. It is important
to keep using it even if you feel well.
If you take too much (overdose)
Immediately telephone your doctor or pharmacist or the Poisons Information Centre
(telephone 13 11 26) or go to Emergency at the nearest hospital, if you think that
you or anyone else may have used too much Atrovent UDV. Do this even if there are
no signs of discomfort or poisoning.
You may need urgent medical attention.
Symptoms of an overdose may include:
fast or irregular heart beat
dry mouth
blurred vision.
While you are using Atrovent UDV
Things you must do
Stop using Atrovent UDV and tell your doctor immediately if you get sudden tightness
of the chest, coughing, wheezing or breathlessness immediately after using Atrovent
UDV.
These may be signs of a condition called bronchospasm.
If you have an Asthma Action Plan that you have agreed with your doctor, follow it
closely at all times.
If you find that the usual dose of Atrovent UDV is not giving as much relief as before,
or you need to use it more often, contact your doctor so that your condition can be
checked.
This is important to ensure your breathing problem is controlled properly.
Continue using Atrovent UDV for as long as your doctor or pharmacist tells you.
Visit your doctor regularly to check on your asthma condition.
Contact your doctor immediately if you experience irritation or a feeling of having
something in the eye or any disturbances with your sight (blurred vision, visual halos
or coloured images) together with red eyes, during or after using Atrovent UDV.
This may mean that you have developed a serious eye condition called narrow-angle
glaucoma. This can happen if the mist gets in your eyes.
Tell any other doctors, dentists, and pharmacists who are treating you that you are
using Atrovent UDV.
If you are about to start any new medicine, tell your doctor or pharmacist that you
are using Atrovent UDV.
If you plan to have surgery, tell your surgeon or anaesthetist that you are using
Atrovent UDV.
It may affect other medicines during surgery.
If you become pregnant while using Atrovent UDV tell your doctor or pharmacist immediately.
Things you must not do
Do not take any other medicines for your breathing problems without checking with
your doctor.
Do not give Atrovent UDV to anyone else, even if they have the same condition as you.
Do not use Atrovent UDV to treat any other complaints unless your doctor or pharmacist
tells you to.
Do not stop using Atrovent UDV or lower the dosage, without checking with your doctor
or pharmacist.
Do not allow the Atrovent UDV mist to enter the eyes.
Things to be careful of
Be careful driving or operating machinery until you know how Atrovent UDV affects
you.
Atrovent UDV may cause dizziness and blurred vision in some people. If you have any
of these symptoms, do not drive, operate machinery or do anything else that could
be dangerous. Children should be careful when performing physical activities.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you
are using Atrovent UDV.
This medicine helps most people with asthma or chronic obstructive bronchitis, but
may have unwanted side effects in a few people. All medicines can have side effects.
Sometimes they are serious, most of the time they are not. You may need medical treatment
if you get some of the side effects.
Do not be alarmed by this list of possible side effects.
You may not experience any of them.
Ask your doctor or pharmacist to answer any questions you may have.
If you get any side effects, do not stop using the Atrovent UDV without first talking
to your doctor or pharmacist.
Tell your doctor or pharmacist if you notice any of the following and they worry you:
headache
dizziness
dry or sore mouth
throat irritation
cough
nausea, vomiting
a change in bowel movements (e.g. constipation, diarrhoea, wind, indigestion, reflux
(an unusual backflow of fluid)).
These are the more common side effects of Atrovent UDV. Most of these are mild and
short lived.
Tell your doctor as soon as possible if you experience difficulty passing urine.
This is a serious side effect that may require medical attention.
If any of the following happen, tell your doctor immediately or go to Emergency at
the nearest hospital:
difficulty breathing or worsening of your breathing problems
spasm of the muscles around the voice box, causing choking
swelling of the throat
fast or irregular heart beat, also called palpitations
pounding heart beat
allergic reaction (shortness of breath, wheezing or troubled breathing; swelling of
the face, lips, tongue or other parts of the body, rash, itching or hives on the
skin)
irritation or a feeling of having something in the eye, red eyes, dilated pupils,
blurred vision, visual halos or coloured images.
The above list includes very serious side effects. You may need urgent medical attention
or hospitalisation.
Tell your doctor or pharmacist if you notice anything else that is making you feel
unwell.
Other side effects not listed above may occur in some people.
After using Atrovent UDV
Storage
Keep your Atrovent UDV in a cool, dry place where the temperature stays below 25°C
and protect from light.
Do not store Atrovent UDV or any other medicine in the bathroom or near a sink.
Do not leave it in the car on hot days or on window sills.
Heat and dampness can destroy some medicines.
Keep it where children cannot reach it.
A locked cupboard at least one-and-a-half metres above the ground is a good place
to store medicines.
Disposal
If your doctor tells you to stop using Atrovent UDV or it has passed its expiry date,
ask your pharmacist what to do with any that is left over.
Product Description
What it looks like
Atrovent UDV is a clear, colourless solution, supplied as Unit Dose Vials containing
1 mL of solution in packs of 30 vials arranged as 3 individually foil-wrapped strips
of 10 unit dose vials, or 10 vials*.
*Not currently distributed in Australia.
Ingredients
Each 1 mL of Atrovent Adult UDV contains 522 micrograms of ipratropium bromide monohydrate
(equivalent to 500 micrograms of ipratropium bromide) as the active ingredient.
Each 1 mL of Atrovent 250 µg/1mL UDV contains 261 micrograms of ipratropium bromide
monohydrate (equivalent to 250 micrograms of ipratropium bromide) as the active ingredient.
It also contains:
sodium chloride
hydrochloric acid
purified water.
Atrovent UDV does not contain lactose, sucrose, gluten, tartrazine or any other azo
dyes.
Supplier
Atrovent UDV is supplied in Australia by:
Boehringer Ingelheim Pty Limited
ABN 52 000 452 308
Sydney NSW
www.boehringer-ingelheim.com.au
This Consumer Medicine Information was revised in
October 2019.
® Atrovent is a registered trademark of Boehringer Ingelheim
© Boehringer Ingelheim Pty Limited 2019
Australian Registration Numbers:
Atrovent 250 µg/1 mL UDV:
AUST R 17909
Atrovent Adult 500 µg/1 mL UDV:
AUST R 58203
Atrovent® UDV
Atrovent 250 µg/1 mL and Atrovent Adult 500 µg/1 mL Unit Dose Vial (UDV)
ipratropium bromide monohydrate
Directions for use
Atrovent UDV are intended for inhalation with suitable nebulising equipment and must
not be swallowed or given by injection.
Diluted solutions should be freshly prepared before use.
At the end of inhalation, leftover solution from the nebuliser bowl should be discarded.
Take the following steps in using Atrovent UDV and if you have any problems, ask your
doctor or pharmacist for assistance.
1. Get your nebuliser ready by following the manufacturer's instructions and the advice
of your doctor.
2. Carefully tear a new vial from the strip. Never use one that has been opened already.
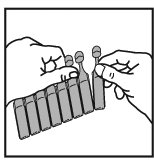
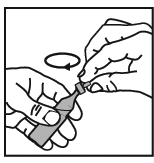
3. Open the vial by twisting off the top, always taking care to hold it in an upright
position.
4. Squeeze the contents of the vial into the nebuliser bowl. If dilution is necessary
this should be carried out using normal saline and as instructed by your doctor.
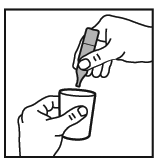
5. Dilute with saline up to a final volume of 2 – 3 mL.
6. Assemble the nebuliser and take the medicine or give it to your child as directed
by your doctor.
7. After nebulisation follow the manufacturer's instructions about cleaning your nebuliser.