Acetolactate synthase, also known as acetohydroxyacid synthase (AHAS), is an enzyme involved in the branched chain amino acid biosynthesis pathway.
Skip to:
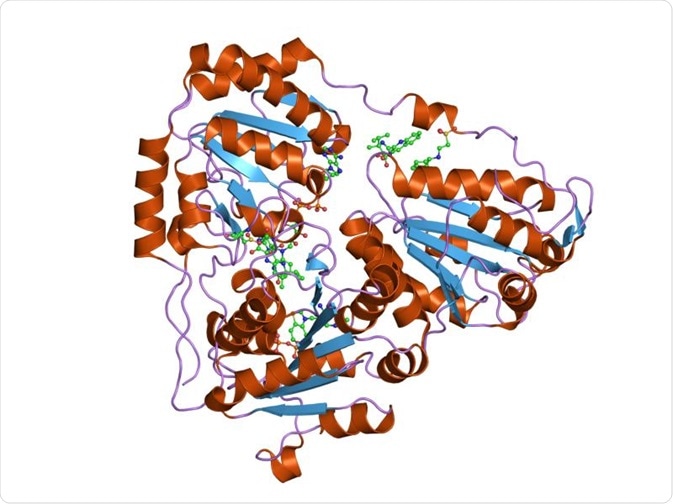
Acetohydroxyacidsynthase catalytic subunit. Credit: Jawahar Swaminathan and MSD staff at the European Bioinformatics Institute.
Where does acetolactate synthase exist?
Acetolactate synthase can be found in plants, fungi, archaea, and bacteria, but not animals. This enzyme is involved in condensing two pyruvate molecules to form acetolactate, or pyruvate and 2-ketobutyrate. This leads to the formation of acetohydroxybutyrate (AHB). In the case of eubacteria, like E.coli or Salmonella typhimurium, three types of acetolactate synthase (AHAS) enzymes are present: AHAS I, AHAS II, AHAS III. However, in E.coli, AHAS II is inactive, while in Salmonella, AHAS III is inactive.
Structure of acetolactate synthase
AHAS is a thiamine diphosphate (ThDP)-dependent enzyme that belongs to the pyruvate oxidase (PO)-like subfamily. The members of this family all share a similar three-dimensional crystal structure. However, the main difference is that AHAS consists of a large subunit and a small subunit, while all the other members consist of only the large subunit. AHAS can condense two pyruvate molecules to form acetolactate that can be subsequently used for the biosynthesis of acetoin.
Acetolactate synthase usually exists as a dimer, but tetramers may be formed when it is crystallized with herbicide. The large subunit consists of three domains–α, β, and γ, where the α domain binds to pyrimidine bases, while the γ domain binds to pyrophosphates. These domains consist of parallel β sheets that are surrounded by α helices. Thiamine diphosphate (ThDP), Mg2+ ions, and flavin-adenine dinucleotide (FAD) are required as cofactors to activate AHAS.
Catalytic mechanism
The reaction catalyzed by acetolactate synthase involves the condensation of two molecules of pyruvate into acetolactate. The first step in this process involves the binding of pyruvate to the active center of acetolactate synthase, followed by the ionization and addition of a carbonyl group to the carbanion of thiamine diphosphate to form a tetrahedral intermediate.
This intermediate subsequently undergoes decarboxylation to release carbon dioxide. The first few steps are common to all the members of the pyruvate oxidase (PO)-like subfamily. However, after the first three steps there is a divergence in the catalytic mechanism of acetolactate synthase among different biological kingdoms and species.
Enzyme subunits
Acetolactate synthase consists of a large and small subunit. If these two subunits are expressed separately and then incubated together for some time, they can constitute a holoenzyme. The small subunit of acetolactate synthase has two functions: activating the catalytic subunit and regulating the enzyme via feedback inhibition of the end products. That is why they have been termed as regulatory units. The size of the small subunits range from 85–170 amino acids and have a sequence identity of less than 25%.
Acetolactate synthase as a target for antimicrobial drug discovery
As acetolactate synthase is present in microorganisms and plants but not animals, it has become a unique target for drug and herbicide discovery. This enzyme is a target for several commercial herbicides, such as sulfonylurea and imidazolinone. These are potent herbicides that selectively inhibit acetolactate synthase. Such herbicides are highly efficacious, and so are only required in small amounts, and have low toxicity to mammals, increasing their importance as biocidal agents.
Given the importance of acetolactate synthase in the metabolism of microorganisms, recent studies are beginning to explore the inhibitors of this enzyme as commercial antimicrobials. Several studies are being conducted to characterize acetolactate synthase from bacteria and fungi, analyze their structure, and provide data on how these can be potentially developed into antimicrobial therapeutics.
Further Reading